United States Pharmacopeia (USP) has announced that the Dec. 1 implementation deadline for USP General Chapters 795, 797, and 825 has been delayed because the stakeholder appeal processes will not be finalized by then. CHA will host a webinar Nov. 12 to assist hospitals working to comply with the standards.
Because General Chapter 800 is not subject to pending appeals, it will become official on Dec. 1. However, because other chapters are under appeal, it will be informational only.
CHA Webinar Nov. 12
CHA will hold a webinar Nov. 12 from 1-3 p.m. (PT) to assist hospitals in continuing their pharmacy sterile compounding construction activities as planned, in order to meet USP standards once they become official. The webinar will include officials from the California Department of Public Health, Board of Pharmacy (BoP), and Office of Statewide Health Planning and Development, who will offer their insights on avoiding lengthy and costly delays.
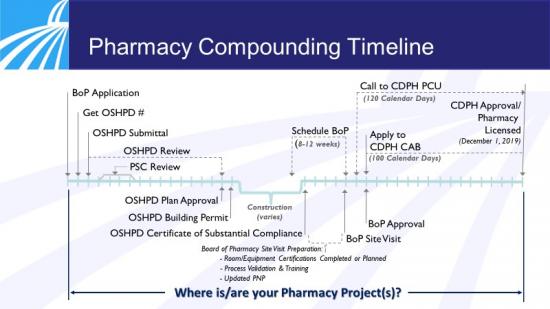
Regulatory Approval Timeline
The timeline below shows the necessary activities and regulatory approvals. Due to the complexity and rigor of the work and approvals, the BoP encourages all hospitals updating their pharmacies to comply with the USP sterile compounding standards to contact the BoP six to eight weeks before they need a BoP site certification visit. Hospitals are also advised to involve the pharmacist in charge in every step of the construction process to avoid costly and timely delays.